Van de Graaf Lab
The research group of Stan van de Graaf is interested in metabolite dynamics and liver-centered organ crosstalk
A plethora of signals comes from the intestine in the form of metabolites produced by the microbiome with food as the source. Different types of food or food components are associated with improved health or with disease. Similarly, dysbiosis, or an aberrant intestinal microbiota composition and function, associates with many diseases. The ultimate goal of my research is to exploit the intestinal-derived signals to prevent or target common metabolic diseases, such as type 2 diabetes, NAFLD and atherosclerosis. Likely, the liver is the key to success as this organ is the first to detect and integrate the signals and plays a dominant role in controlling the spatial and temporal signaling of gut-derived metabolites.
Specifically, we aim to stimulate specific signals by modulating transport and detection pathways in the liver. To this end we generate novel tools to specifically modulate hepatic metabolite uptake/sensing in vivo. We use a variety of techniques, including (live cell) confocal microscopy, FRET imaging, biochemical characterization of transporter trafficking, cellular transport/enzymatic assays and high throughput screens with inhibitors/shRNA/gRNAs pools in combination with in vivo studies using transgenic mice, disease-mimicking cell systems and isolated organ perfusion.
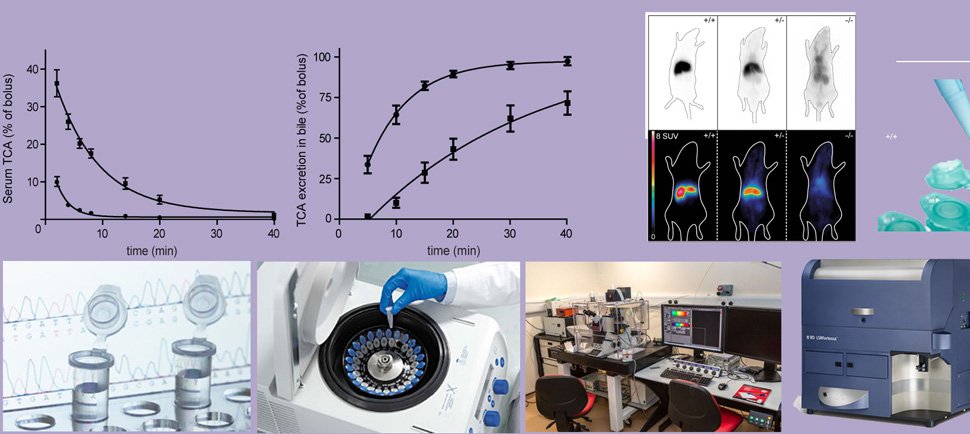
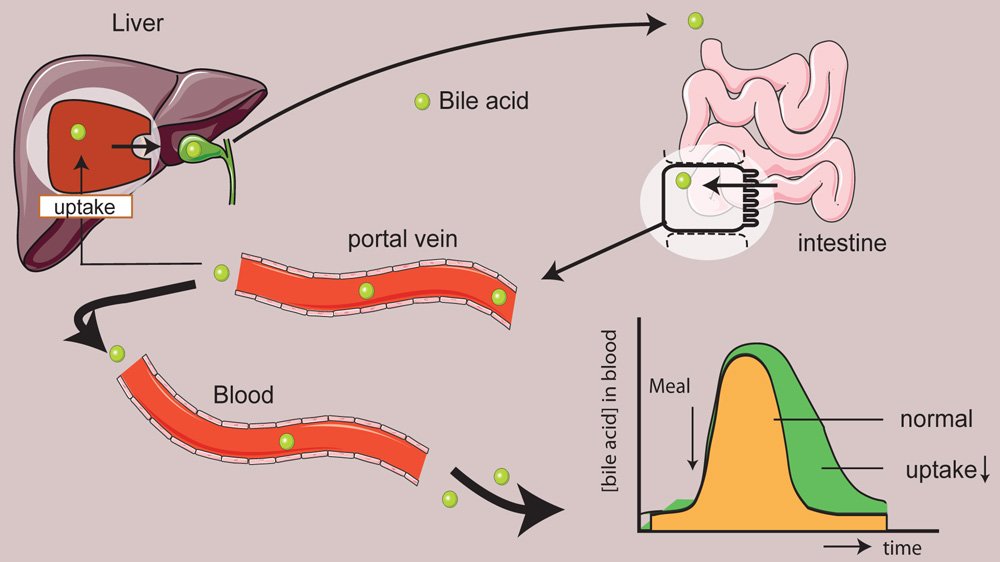
Recent proof-of-concept data was obtained by targeting the bile acid transporter NTCP. Genetic and pharmacological targeting of this liver-specific transporter resulted in prolonged bile acid signaling leading to enhanced release of GLP1, increased energy expenditure, reduced intestinal lipid uptake and dampening of inflammation. For decades bile acids were believed to fulfil a rather passive role in metabolism as powerful detergents. Since the discovery of specialized bile acid-sensing proteins, such as such as the farnesoid X receptor (FXR) and the G protein-coupled bile acid receptor (TGR5, also GPBAR1 or M-BAR), it has become clear that bile acids also have beneficial signaling effects which could be exploited to improve human health. We are only starting to understand the role of bile acids (and its dynamics) in energy homeostasis and my group is studying this process in great detail. Modulation of bile acid dynamics/localization is a powerful tool for this.
Furthermore, shifting bile acids away from the liver into the peripheral circulation dampened hepatic damage due to cholestatic liver diseases (chole-stasis = impaired bile flow). NTCP inhibition dampens cholestatic liver injury by lowering hepatocellular bile salt accumulation and by reducing biliary toxicity. Cholestasis constitutes a major cause of liver failure, since accumulated BSs are cytotoxic. This plays a role in multiple cholestatic liver diseases, including Primary Sclerosing Cholangitis, Primary Biliary Cholangitis and progressive intrahepatic familial cholestasis (PFIC). As a related research line we aim to open new areas of diagnosis, research and future treatment of cholestasis
In-house collaborative projects
Together with Dr. Ulrich Beuers we aim to elucidate the etiology of IgG4 Related Disease